Fluoride Shuttle Battery
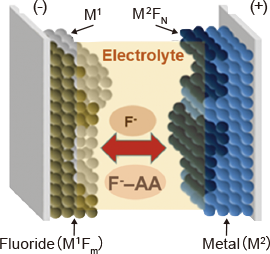
The research in this group is focusing on the development of fluoride shuttle batteries (FSBs) as beyond-lithium–ion, innovative, rechargeable battery systems working on the principle of the reversible redox transformation between a metal and its fluoride counterpart at both the negative and positive electrode under fluoride–ion shuttling. Control over the multi-electron redox reactions involving more or less insulating metal fluorides is difficult to achieve without breakthrough technologies in relation to numerous aspects of this approach.
The close ties between this research group and the Advanced Analysis Technology team has enabled the research to progress to establishing both all-solid and liquid-based FSBs.
Features and Advantages
- High energy densities as a result of multi-electron negative and positive electrode reactions
- Diverse combinations of negative and positive electrode materials
- High fluorine overvoltage, promising a high level of safety
- Unprecedented anion-based battery systems based on innovative cell operation concepts
R&D Themes
- Development of solid and liquid electrolytes with high fluoride–ion conductivities
- Advanced technologies for tailoring low fluoride–ion-conductive negative and positive electrode materials to high-capacity battery operation
- Control over the interfacial material, charge transfers, and heterogeneous reactions
R&D Strategies
- Solid and liquid electrolytes ensuring both high fluoride–ion conductivities and high redox stabilities
- Balance between fluoride–ion conductivity and electron-accepting capability for reactions of the active materials, along with control over the solubility of active materials in the liquid electrolyte
- Explication of the reaction mechanism and dynamics using state-of-the-art analysis technologies
Research Results
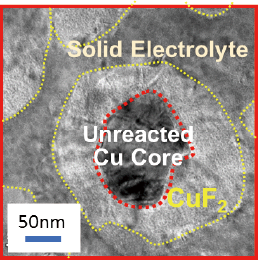
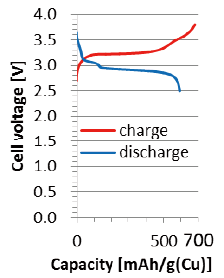
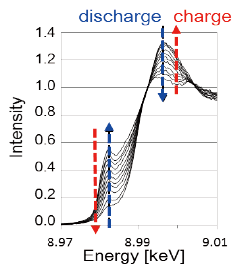
Successful operation of a low fluoride–ion-conductivity CuF2/Cu positive electrode by sophisticated nano-interfacial control of the solid-state reaction, as confirmed by operando XAFS analysis.
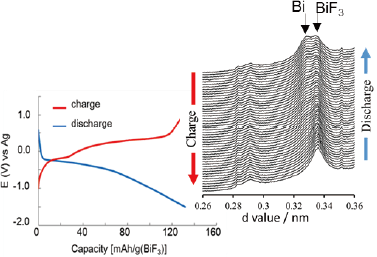
Reversible charge–discharge of a BiF3 composite electrode with a novel liquid electrolyte, as confirmed by operando XRD analysis.
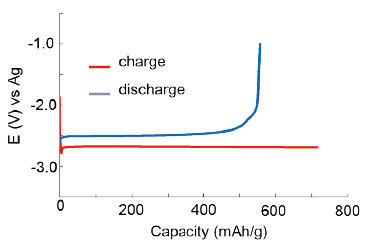
Activation of an AlF3-based negative electrode by complementary compositional and structural control of the active material.
